ABSOLUTE RISK vs RELATIVE RISK REDUCTION
In summary ….
- Relative risk reduction is valuable in marketing and product approval, but absolute risk reduction is more useful in the individual’s decision about whether to be vaccinated
- Real world data from UK suggest a higher rate of infection among the vaccinated than unvaccinated, although there appears to be some reduction in hospitalisations and deaths
- Number needed to vaccinate (NNV) recognizes the exposure of individuals to vaccination adverse effects to reduce COVID-19 related events such as infection, hospitalisation and death
- Multiple authors have raised criticisms of the UK-derived data, including possible undercounting or overcounting of unvaccinated vs vaccinated groups, as well as criticisms over various irregularities in the Pfizer clinical trial
Claim 17: Pfizer’s own data proves the vaccine only reduces the chance of infection by 0.73 percent, not 90-plus percent it claimed; no one in the trial was actually proven to have the virus
NH: “Dr Canaday came up with the rather low efficacy figure of 0.73 percent by looking at how many people in each group of the trial got infected – 0.77 percent of the placebo group, and 0.04 percent in those who got the jab. The first figure minus the second equals 0.73.
DrPC: The initial data from the Pfizer clinical trials involved 21,728 subjects in the placebo group and 21,720 subjects in the vaccine group. Of these, 162 in the placebo group developed COVID-19 (defined as a positive PCR test and one or more symptoms), as did 8 in the “active agent” group (= vaccinated). Thus, the incidence of COVID-19 was 162/21,728= 0.75% in the placebo group and 8/21,720= 0.037% in the active agent (= vaccine) group. Subtracting one from the other gives 0.75%-0.037%= 0.71% reduction.
The relative difference in the groups is (0.75%-.037%)/0.75%= 95% in favour of the vaccinated group, and that’s where the claim of “95% efficacy” came from. That is what is termed “Relative Risk Reduction,” precisely because relative to the unvaccinated group, there was a 95% reduction in COVID-19, as defined in this way.
On the other hand, 21,566 subjects in the unvaccinated group, or 99.25%, never developed COVID-19. In the vaccinated group, 21,712 subjects, or 99.96% never developed COVID-19 during the 3.5 months of the trial as stated in the interim report. The reduction from 0.75% in the unvaccinated group to 0.037% in the vaccinated group represents the “Absolute Risk Reduction,” precisely because on an absolute basis, there was a 0.71% reduction in likelihood of developing COVID-19 when the 3.5-month trial period was referenced in the preliminary report.
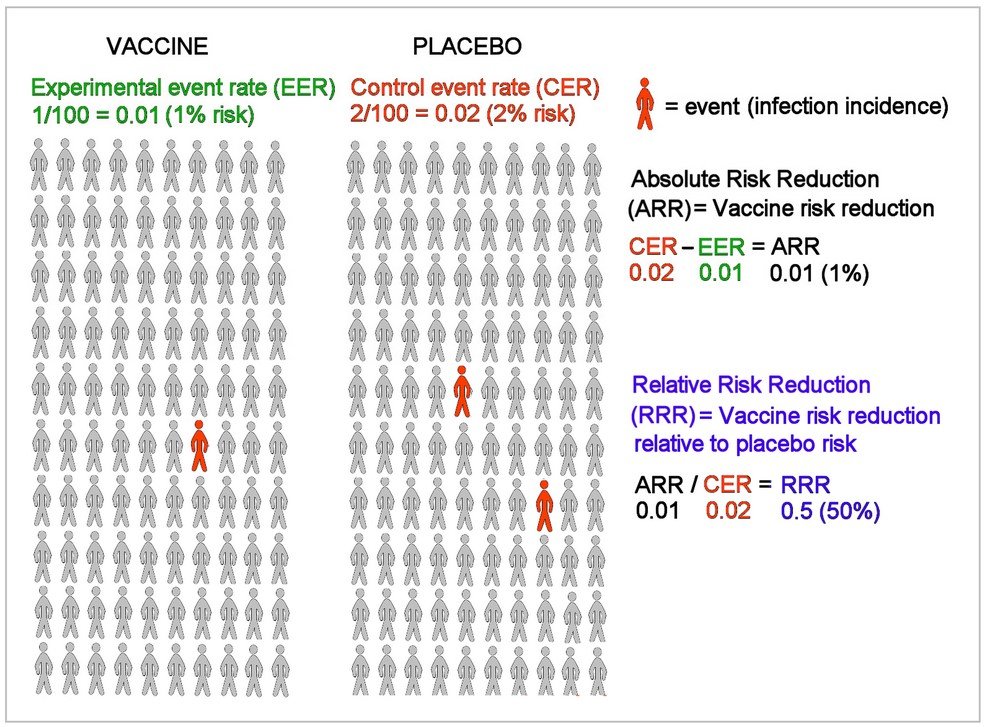
An example below uses a 50% relative risk reduction and confirms a 1% absolute risk reduction to illustrate how these two calculations differ (R1):
NH: “Dr Petousis-Harris said this is not how efficacy is calculated.
DrPC: That depends on whether you are trying to get a medical intervention approved by a regulatory agency and attempting to convince the public in media and government pronouncements, or whether you are Joe Public who just wants to know what his chances are of getting COVID-19 if he decides to take or not to take the jab. I believe that most people reading this post are more interested in the second enquiry than the first. (R1)
NH: “We estimate vaccine efficacy by comparing the rate of disease in the vaccinated participants compared with the rate of disease in the unvaccinated.
Doing that, it’s clear people in the trial were about 19 times less likely to contract COVID-19 if they were vaccinated than if they weren’t.
DrPC: The mathematically correct way to term that is: the vaccinated were one-twentieth as likely to contract COVID-19 as the unvaccinated. That’s the Relative Risk Reduction that Dr Petousis-Harris is talking about. True, but not very impressive when the overall likelihood of developing the disease is reduced by only 0.71%.
Relative risk reduction is valuable in marketing and product approval, but absolute risk reduction is more useful in the individual’s decision about whether to be vaccinated
NH: “Dr Canaday said the trial counted a person as having the virus if they had at least one symptom and a positive PCR test, casting doubt on whether anyone was actually infected “given what we know about the issues with PCR testing.”
DrPC: We’re not able to determine who got “infected” because antibody conversion with or without additional research studies to detect cellular immunity after exposure to the SARS-CoV-2 virus was not tested (or at least was not publicly disclosed). “Infected” means that our immune system has “seen” the virus, and mounted a response to it.
All we see from the Pfizer press release and the initial published trial data is a comparison of those who became “symptomatic” with COVID-19, defined as a positive PCR test and one or more symptoms.
Pfizer listed “symptoms” as: “at least one of the following symptoms: fever, new or increased cough, new or increased shortness of breath, chills, new or increased muscle pain, new loss of taste or smell, sore throat, diarrhea, or vomiting”. (R2) Incidentally, loss of sensation of smell is not specific for COVID-19. Post-viral olfactory dysfunction is a well-established clinical entity seen with many other viral syndromes. (R3)
The rest of these symptoms are also seen with many viral illnesses; none are specific enough to COVID-19 to merit the label of “suspected COVID-19” and could mean that any respiratory or other viral illness could become labelled as “COVID-19” when a PCR test is positive!
The study also reported 10 hospitalisations during the trial, 9 in the unvaccinated group and 1 in the vaccinated group. The numbers however are too small to be statistically significant. No one died during the initial period when early results of the clinical trial were reported.
To be fair, the reported six-month data from the Pfizer phase III clinical trial appeared recently and continued to show at least a 91% relative risk reduction. (R4) With passage of time, 81 of 21,642 in the vaccinated group (= 0.37%) developed symptomatic COVID-19, whereas 873 of 21,689 did in the unvaccinated group (= 4.0%).
This represents an absolute risk reduction of 3.6%. In other words, 99.6% were free of COVID-19 with the vaccine, and 96% were free of COVID-19 without it. Protection began 11 days after the second dose, and then declined by about 6% per month after that in this six-month report. In this instance, the number of deaths where all causes were included did not differ in the 6-month results. (R4) Issues with the reliability and integrity of the Pfizer data will be discussed below.
Evaluating real world data
Real world data now available from Public Health England and its successor, UK Health Security Agency, suggest that the decline in protection in the era of the delta variant is considerably higher, especially for the older age groups, but even for those in the middle-age group. For those aged 40-49, in fact, the data show a negative efficacy for reducing infection. In other words, more vaccinated than unvaccinated acquire infection based upon PCR testing– 1731 vs 773 per 100,000, and this continues for older age groups as well: (R5, R6)
Current data still show benefit in reducing hospitalisations, although to a lesser degree than one would expect from the “95% efficacy” trope so widely published— 6.5 vs 27 hospitalisations per 100,000 comparing vaccinated to unvaccinated, and this reflects a 76% relative risk reduction or efficacy:
However, if we look at the absolute risk reduction, we see a reduction from 0.027% to 0.0065% risk of hospitalisation if one is vaccinated
The difference in mortality rates from vaccinated vs unvaccinated also remains, although it again is not so impressive as one might have expected from the “95%” claim. We compare 0.5 deaths to 2.0 deaths per 100,000 in the vaccinated vs the unvaccinated, or 75% relative risk reduction or efficacy:
Again, if we look at the absolute risk reduction, we see a reduction from 0.002% in the unvaccinated to 0.0005% risk of death if one is vaccinated. In other words, from very, very small to extremely small risk. Results of course will vary for different age categories.
All these figures represent the data over a 4-week period ending in October 2021 and could be assessed over a longer period to magnify the analysis. Data from Israel where the delta variant was dominant confirms waning efficacy of the Pfizer vaccination regimen, with efficacy falling to 39% at 6 months. (R7, R8)
How to extend these results over a longer period during which the vaccination remains effective is therefore challenging: there is concern that the current vaccination regimen wanes in efficacy after 6 months in the USA where 77% of trial participants were studied even before the delta variant became dominant.
Even the “six-month safety and efficacy” study referred to above only contained 7% of subjects who had reached 6 months of follow-up. (R7)
Concerns also remain about what exactly is the meaning of a death within 28 days of a positive COVID-19 test for someone who may have been admitted with an unrelated diagnosis, and test positive when exposed to others in the hospital with active COVID-19.
There also remains a concern whether, as in the USA, the definition of “unvaccinated” extends to those who have received only the first vaccine dose, and even the second dose, until 14 days have passed. (R9) (Absolute numbers for the partially vaccinated are given in the UK statistics, but we do not have a rate per 100,000).
Real world data from UK suggest a higher rate of infection among the vaccinated than unvaccinated, although there appears to be some reduction in hospitalisations and deaths
Another look at the trial results: NNV
We’ve seen the data for the Relative Risk Reduction and the Absolute Risk Reduction (ARR). There is another way to encounter the implications of these results: the Number Needed to Vaccinate (NNV) to achieve a desired result. (R10) In the case of “getting COVID-19” as defined by the PCR test and one or more symptoms, it is the inverse of the ARR. From the initial trial data above, this would be 1/0.0071= 140 subjects to vaccinate in order to prevent one “case.”
Considering data from the follow-up publication about the Pfizer clinical trial, the NNV is 1/0.036= 28 subjects to vaccinate in order to prevent one “case.”. The real-world data from Israel tell a different story, with ARR= 0.46% and NNV is 1/.0046= 217 subjects to vaccinate in order to prevent one “case”. (R10) This means that these individuals would be exposed to the process of becoming vaccinated, along with any attendant adverse effects, and would derive marginal personal benefit from doing so.
If we calculate NNV from the “real world” UK data regarding hospitalisations and regarding deaths, the NNV works out to 1/ (0.00027-0.000065) = 4878 subjects to vaccinate in order to prevent one hospitalisation, and 1/ (0.00002-0.000005) = 66,667 subjects to vaccinate in order to prevent one death from COVID-19 as defined in the UK statistics during the 4-week period covered.
Again, these individuals would be exposed to the process of becoming vaccinated, along with any attendant adverse effects, and would derive very marginal personal benefit from doing so.
Number needed to vaccinate (NNV) recognizes the exposure of individuals to vaccination adverse effects in order to reduce COVID-19 related events such as infection, hospitalisation and death
Trial subject groups not representative of the population at risk
It is known that the Pfizer clinical trial enrolled mainly healthy individuals with fewer subjects over 65 than may have been proportionately most susceptible to morbidity and mortality from COVID-19. To emphasise this point, the overall mortality in the Pfizer trial was calculated to be only 4% of overall expected USA mortality during the period of the study, and even so, it was one-fifth the mortality rate found in the Moderna trial (another mRNA-based formulation). (R11)
What then can be concluded about the age groups most at risk from mortality from COVID-19, and those most likely to have co-morbid conditions, to whom the vaccine’s benefit should be most directed?
Irregularities in study design
Criticism has also been raised about whether the UK Office for National Statistics may be undercounting the number of unvaccinated, and therefore inflating cases, hospitalisations and deaths in that group. (R12) The authors provide a detailed analysis of the data provided, including additional suggestions that the deaths in the vaccinated group may also have been underestimated, particularly given the unexpectedly high mortality after the first doses were given. (R13)
Peter Doshi has raised concerns that there were 20x as many cases of “suspected COVID-19” between the active group and the placebo group in the Pfizer trial. (R14) These were individuals who had “flu-like” symptoms that could have represented COVID-19 but tested negative by PCR, or so goes the argument. On the other hand, there are many more respiratory viruses that historically have caused such clinical presentations, including RSV, adenoviruses, other species of coronaviruses, and the influenza virus itself.
In fact, if the PCR test had a false-positive rate of only 5%, and one out of 20 illnesses labelled as suspected COVID-19 became labelled as definite COVID-19 due to a false positive PCR test for the SARS-CoV-2 virus, then it is possible that ALL influenza-like illnesses during the trial period were associated with viruses other than the SARS-CoV2 virus!
Clinical trial integrity
A recent investigative report published in the prestigious British Medical Journal provided evidence of multiple regularities at several of Pfizer’s vaccine testing sites in Texas during the phase III clinical trial (R15):
“A regional director who was employed at the research organisation Ventavia Research Group has told The BMJ that the company falsified data, unblinded patients, employed inadequately trained vaccinators, and was slow to follow up on adverse events reported in Pfizer’s pivotal phase III trial. Staff who conducted quality control checks were overwhelmed by the volume of problems they were finding. After repeatedly notifying Ventavia of these problems, the regional director, Brook Jackson, emailed a complaint to the US Food and Drug Administration (FDA). Ventavia fired her later the same day. Jackson has provided The BMJ with dozens of internal company documents, photos, audio recordings, and emails.”
Pfizer’s briefing documents submitted to the FDA, which eventuated in approval of its vaccine the next day, had failed to acknowledge the myriad problems at the Ventavia sites, where none of the required site inspections had actually been carried out.
Pfizer also offered their product to the placebo group after 13 March 2021 (the cut-off date for 6-month data submitted to the FDA), and 93% of trial participants decided to “exit” early from any sustained follow-up that a truly randomised, placebo-controlled clinical trial would entail. (R8)
Multiple authors have raised criticisms of the UK-derived data, including possible undercounting or overcounting of unvaccinated vs vaccinated groups, as well as criticisms over various irregularities in the Pfizer clinical trial
NH: “PCR testing is between 95 and 100 percent accurate, according to the Ministry of Health.”
DrPC: As just discussed, 95% accuracy could have allowed the 5% false positives discussed above under “Irregularities in study design”, meaning that we could have been looking entirely at respiratory illnesses during the Pfizer trial, none of which were causally associated with SARS-CoV2. Given the concerns as to whether the SARS-CoV-2 virus has even been isolated in the first place (R16) and that restrictive measures passed by several government entities to date have thus been successfully challenged as baseless (R17, R18), the matter of relative risk reduction vs absolute risk reduction may have become moot.
The topics of false positive PCR tests and whether the PCR test is even fit for diagnosis are discussed in responses #1 and #9 respectively. The matter of different testing methods used for PCR testing in the unvaccinated vs the vaccinated will be addressed in response #2.
Summary and Implications
Relative risk compares the placebo group vs the vaccinated group as to how effective the vaccine is in reducing the outcome measured, and this is useful in promotion and for seeking approval in regulatory agencies. Absolute risk reduction is what’s useful for the person considering vaccination, where the prevalence of the actual disease and the outcome measured are considered. Absolute risk reduction in the setting of the Pfizer vaccine is much less impressive (at less than 1%) than the 95% figure that is promoted in the popular press and repeated in government pronouncements.
The Pfizer clinical trial required participants to have only one or more symptoms commonly seen in many viral respiratory or other illnesses, in combination with a positive PCR test for SARS-CoV-2. Extensive criticisms of the applicability of PCR testing in the clinical setting, the high degree of false positives where a high “cycle threshold” for detection of RNA genetic material is used, among others have been addressed in responses #1 and #9 and will further be addressed in response #2.
Real world experience with the Pfizer vaccination programs in Israel and United Kingdom have shown that vaccinated individuals may actually have a higher rate of infection by SARS-CoV-2 than the unvaccinated, although published studies still show some reduction in hospitalisations and deaths.
Viral load and viral transmission to household contacts however have been similar in both vaccinated and unvaccinated (25% vs 23%) even though protection of the vaccinated individual from COVID-19 complications may be better for up to 2 months if trial results can be trusted. (R19) This challenges the notion that vaccination will “protect your whānau and others from COVID-19.”
As we consider what we can conclude from the Pfizer clinical trial, we must take note of the substantive criticisms around study design and especially study execution where traditional protections around data integrity appear to have been violated.
There may be less here than meets the eye, and arguably, less trustworthy conclusions from the Pfizer trial upon which to base public policy than we have been led to believe.
References:
R1: Outcome reporting bias in COVID-19 mRNA Vaccine Clinical Trials. Medicina 2021; 57: 199
R2: Safety and Efficacy of the Pfizer vaccine (initial). https://www.nejm.org/doi/full/10.1056/nejmoa2034577
R3: Post-viral Anosmia Did Not Begin with COVID-19! https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8067782
R4: Safety and Efficacy of the Pfizer vaccine through 6 months. https://www.nejm.org/doi/full/10.1056/NEJMoa2110345
R7: Waning immunity of the BNT162b2 vaccine; A nationwide study from Israel. https://www.medrxiv.org/content/10.1101/2021.08.24.21262423v1
R8: Does the FDA think these data justify the first full approval of a Covid-19 vaccine? https://blogs.bmj.com/bmj/2021/08/23/does-the-fda-think-these-data-justify-the-first-full-approval-of-a-covid-19-vaccine
R9: CDC changes definition of “unvaccinated.” https://www.cdc.gov/coronavirus/2019-ncov/vaccines/fully-vaccinated.html
R10: COVID-19 vaccine efficacy and effectiveness. https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(21)00069-0/fulltext#bib11
R11: Moderna and Pfizer vaccine arm deaths only 20% and 4% of expected USA mortality rates. https://childrenshealthdefense.org/defender/discrepancies-modernas-fda-report-demand-answers/?utm_source=twitter&utm_medium=defender
R12: Fenton and Neil_ Assessing all-cause mortality in the vaxxed vs unvaxxed. https://probabilityandlaw.blogspot.com/2021/10/comparing-all-cause-mortality-rate-by.html
R13: Discrepancies and inconsistencies in UK Government datasets. https://www.researchgate.net/publication/355437113_Discrepancies_and_inconsistencies_in_UK_Government_datasets_
R14: Pfizer and Moderna effectiveness- Peter Doshi. https://blogs.bmj.com/bmj/2021/01/04/peter-doshi-pfizer-and-modernas-95-effective-vaccines-we-need-more-details-and-the-raw-data/
R15: Covid-9: Researcher blows the whistle on data integrity issues in Pfizer’s vaccine trial. https://www.bmj.com/content/375/bmj.n2635
R16: The COVID-19 Fraud: War on Humanity, https://drsambailey.com/2021/11/11/the-covid-19-fraud-war-on-humanity
R17: Portugal court ruling. https://www.theportugalnews.com/news/2020-11-27/covid-pcr-test-reliability-doubtful-portugal-judges/56962
R18: Austrian court ruling. https://greatgameindia.com/austria-court-pcr-test/
R19: Community transmission and viral load kinetics of the SARS-CoV-2 delta variant in vaccinated and unvaccinated in UK. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00648-4/fulltext#seccestitle160