Post Vaccine Symptom Check – A Self-Fulfilling Prophecy?

NZ experts are constantly reassuring us that the Covid-19 injection is ‘safe and effective’. This is despite the original Pfizer trial only having 2 months of safety monitoring data, whistle-blowers calling into question the reliability of the clinical trials and mounting evidence of harm in worldwide monitoring databases. In addition, there is essentially no Pfizer control group to study medium and long term safety as the participants were all offered the option of vaccination not long into the trial.
The public are increasingly concerned about sudden deaths, illnesses and recurrent covid and other infections they are witnessing in injected family members and friends.
The reassurances we are being given are not coming from gold-standard double-blind, randomised, placebo-controlled trials but rather from ‘real world data’.
One of our concerns with the use of real world data (post marketing surveillance) is that it is passive and only a small percentage of potential adverse events ever get reported – Medsafe expects a reporting rate of about 5%.
In order to bolster safety claims, NZ authorities (Medsafe) have done some proactive surveillance in the form of the Post Vaccine Symptom Check. This is similar to the V-Safe data gathered in America.
There is a superficial report of the results on the Medsafe website, however the raw data, (which should always be made available in research trials and was until 2020) does not seem to be able to be obtainable.
The first PVSC campaign took place from 27 August to 5 October 2021 and surveyed people who had received dose 1 or dose 2 of the Pfizer COVID-19 vaccine.
The second survey ran from 14 December 2021 to 31 October 2022, and text messages were sent to random samples of people who had received a booster dose.
Participants who answered YES to having experienced an adverse event/reaction were asked to complete a survey to provide more information about the event.
319,136 (41% of people who were sent a text) responded. Thirty-two percent of these respondents reported that they experienced at least one adverse event/reaction to the vaccine. Ten percent of people who participated in the booster 1 campaign and 4% who participated in the booster 2 campaign reported missing work or other daily activities.
The first survey ran for just under 6 weeks while the second survey ran for 10.5 months.
The most frequently reported adverse events were injection site reaction, headache, muscle and body aches, joint pain, chills, fatigue and tiredness.
The conclusion was:
“The profile of reported events to PVSC for the Pfizer (Comirnaty) COVID-19 vaccine is similar to that reported in clinical trials and from post-marketing surveillance in New Zealand and overseas. Based on this data, we have not identified any new safety concerns.”
The essence of this was stated twice in bold type in the report. This conclusion, however, needs further assessment.
What Were They Actually Looking For?
It is not clear from the information on the Medsafe page exactly what questions were asked and what information was sought.
However, an OIA has managed to get some information.
It appears to be a case of ‘you will find what you are looking for’ and ‘you shall not find that for which you do not look’.
The text was sent 6 days after the dose of the COVID-19 vaccine. It is not obvious in the write-up on Medsafe’s website what the time frame for being able to answer was. Did they only accept answers that same day, or a week later, a month later, 3 months later? Was there a date post-injection after which the responses were not accepted or counted?
Participants were asked if they had any side effects from the vaccine. – ‘Yes’, ‘No’ or ‘Stop’ were the possible responses.
The public (and doctors) had been pre-primed as to what constituted a ‘side effect’ – headache, injection site pain, fever, fatigue, body aches.
According to the extensive and constant marketing, even these adverse events were rare, mild and short-lived. The vaccines were safe. Despite Pfizer’s own post-marketing data listing a range of serious adverse events, the advertising suggested that the side effects did not, and could not, include anything remotely serious.
If ‘Yes’ was selected, then another menu appeared with a list of options. The questions the survey asked are at the end of this article, as provided by OIA.
It states in the Medsafe FAQ answers that “The Ministry of Health will not follow up on any survey responses.” Presumably this means the MoH didn’t plan to contact the respondent or the respondent’s health care practitioners to find out more information or clarify any comments they might have written in the “Other” box.
It is interesting that heart attack, blood clots, stroke, Guillain Barre syndrome, shingles, stillbirth, Bell’s palsy, sudden death and many other conditions weren’t on the list of boxes to tick. What happened if a person experienced one of these and wrote it in the ‘Other’ box? Was that counted and where is that reported?
We note the only question related to pregnancy was “Are you pregnant?” No questions about the outcome of that pregnancy and whether it ended in miscarriage, stillbirth, premature birth, congenital abnormality or a healthy full-term baby.
What Else Is Being Studied?
In contrast to this public-facing and reassuring post-marketing surveillance, behind the walls of academia, according to Dr Helen Petousis-Harris in a Science Media Centre article, the whole population of NZ is being quietly monitored for a lengthy list of life-threatening conditions.
“How was the figure of around 300 million people being monitored arrived at?
It is an estimate based on the number of people each site is working with. Some sites are monitoring just a portion of the country’s population while others like the New Zealand site will monitor the entire population. It is also anticipated that new sites will join the collaboration.”
Before getting into the Global Vaccine Data Network (GVDN) and what they’re up to, it is important to consider that in Feb 2021 the government was aware that there would be a significant burden of adverse events. In this OIA released document the authorities are busy calculating whether it is cheaper to provide time off work for all border workers to be injected or pay for a week off work for the up to 1.1% who would suffer a severe or serious adverse event!

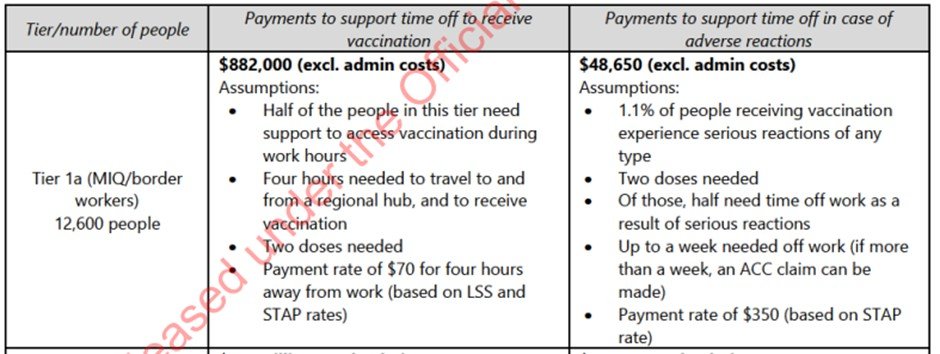
Were New Zealanders informed that 1.1% of people receiving vaccines were expected to suffer a serious adverse event? Would New Zealanders have accepted this much collateral damage had they been informed?
The GVDN is looking at the following lengthy list of conditions and has called them AESIs. (Adverse Events of Special Interest). These are the things that the government was aware of in Feb 2021 as the rollout started. ‘Special Interest’ to WHO we might ask? Not the people or health authorities of NZ.
Adverse Events of Special Interest Monitored by GVDN
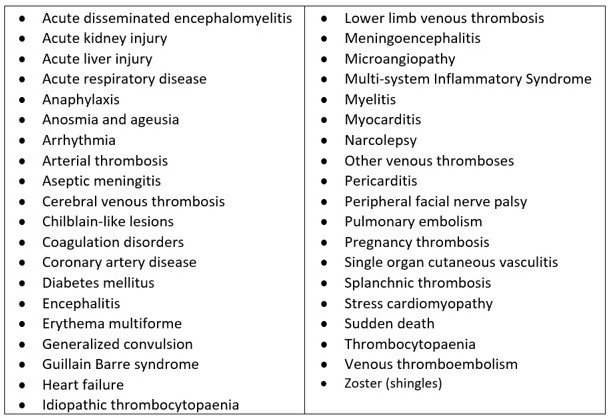
We wonder if people had been asked to fill in a survey with this list of tick box possibilities how the results would look.
NZDSOS contends that there would be large numbers of these events occurring in the days and weeks following vaccination. It is likely that our government knows exactly how many sudden deaths there have been shortly following vaccination, as every New Zealander is on the Covid Immunisation Register (CIR) and it can’t be that hard to match the record of deaths with the vaccination record.
We have been asking questions and pointing out inconsistencies for over 2 years with no response from the authorities. We need your help getting the answers all Kiwis deserve.
Please join us in demanding the answers – from politicians, police, mainstream media, regulatory authorities, family doctors, hospital specialists, coroners and anyone else you can think of.
Post Vaccine Symptom Check Survey
Kia ora,
The Ministry of Health would like to ask you about your recent COVID-19 vaccination. Did you experience any side effects in the days after your COVID-19 vaccination?
Reply YES if you did
Reply NO if you didn’t
Reply STOP if you don’t want to take part
Kia Ora,
We are asking people about their tamaiti (child) who has had a recent COVID-19 vaccination so we can understand their experience with the vaccine.
Did Allie experience any side effects in the days after their recent COVID-19 vaccination?
Reply YES if you did
Reply NO if you didn’t
Reply STOP if you don’t want to take part
The Ministry of Health
Thanks for letting us know about Allie’s side effects. Please complete this survey to provide more detail on their side effects and help us understand their experience with the Pfizer COVID-19 vaccine in Aotearoa New Zealand.
The Ministry of Health
If Yes was selected:
Select all the side effects you experienced after your recent vaccination:
Injection site reaction (pain, redness, swelling, itching at or near the injection site)
Fever/high temperature
Rash (Please describe below)
Headache, muscle/body aches, or joint aches/pain (Please describe below)
Chills (shivering or feeling cold)
Stomach symptoms (Please describe below)
Fatigue or tiredness
Other (Please describe below)
Rash – Conditional Questions
When did the rash start?
Within 1 hour after vaccination
Within 1 day after vaccination
More than a day after vaccination
How long did the rash last?
Less than 30 mins
30 mins to 24 hours
More than 24 hours
Headache/Stomach – Conditional Questions
Headache, muscle/body aches, or joint aches/pains.
Please select all that apply:
Headache
Muscle/body aches
Joint aches/pains
Stomach symptoms.
Please select all that apply:
Nausea
Vomiting
Diarrhoea
Stomach pain
Did you take any medicines to ease your symptoms (eg: paracetamol or ibuprofen)?
Yes
No
Did you go to a doctor for your symptoms?
Yes
No
Did any of the symptoms you reported cause you to miss work, study, or normal daily activities?
Yes
No
How many days did you miss?
Less than 1 day
1 day
2 days
3 days or more
Are you pregnant?
Yes
No
Unknown
Do you have any long-term medical conditions?
Yes
No
Please select which conditions you have:
Autoimmune conditions (e.g. arthritis)
Alcohol or other drug addictions
Asthma
Cancer
Chronic pain
Chronic obstructive pulmonary disease
Diabetes
Gout
Heart disease
Mental health condition
Obesity
Other (Please describe below)
Thanks for taking part in this survey. The information you provide is confidential and is protected by the Privacy Act 2020 and by the safeguards we have in place.
Remember this is a survey only and your answers will not result in a medical response to your situation. If you have concerns about your health since your vaccination, ring the Healthline at 0800 358 5453 or speak to your healthcare professional.
Results from the survey will be published on the Medsafe website at Medsafe.govt.nz as the survey progresses.





