What Is a Case of Covid-19?

Susan Pockett, MSc PhD, a supporter of NZDSOS has provided us her analysis on what constitutes a case of Covid-19.
Jan 2022
In a radical departure from long-‐established medical practice, the World Health Organization decreed in 2020 (1) that the number one criterion necessary for diagnosis of a ‘confirmed case’ of covid 19 is a positive NAAT (Nucleic Acid Amplification Test) for the virus SARS‐CoV-2. Given such a positive NAAT, a ‘confirmed case’ can be diagnosed in the absence of any clinical symptoms.
It is therefore of considerable importance that politicians, everyone concerned with advising politicians and/or ruling on the legality of their mandates pertaining to the virus – and indeed everyone affected by those mandates – should have a reasonably sophisticated understanding of the mechanisms underpinning and the reliability of the most common NAAT used to detect SARS‐CoV‐2.
At present the most widely used such test is a reverse transcription quantitative real‐time polymerase chain reaction (RT-‐qPCR) test (2,3) – commonly known as a PCR test. The aim of
the present article is to provide readers with no previous knowledge in this field (except perhaps a basic awareness of the general structure of RNA and DNA, such as might be obtained from Wikipedia) with such an understanding of the mechanisms and pitfalls involved in the PCR test used to diagnose SARS‐CoV-2.
How do PCR tests for covid work?
SARS‐CoV-2, like its predecessors SARS‐CoV-1, MERS and various kinds of common cold virus, is a beta coronavirus. At present there is room for reasonable debate about both its origin (4) and its novelty (5) – but not about its existence, or the fact that it consists of an RNA genome packaged in a capsid made of proteins and lipids. The PCR test officially diagnostic of ‘a case’ of SARS-CoV-2 aims to detect and quantify one or more of several viral genes – short RNA base sequences that are found in the SARS-CoV-2 genome, and (it is assumed, despite the impossibility of proving a negative) only in the SARS‐CoV‐2 genome.
This PCR test proceeds by way of a number of quite complex steps, which can be summarised as follows:
Step 1: Clean out everything that is not RNA from the swab sample.
This is a vitally important step, for a number of reasons. For example, if extraneous DNA is not completely removed at this point – perhaps DNA from broken-up human cells in the nasal mucous – the polymerase chain reaction in Step 4 of the test could amplify that irrelevant DNA, as well as/instead of the diagnostic cDNA generated in Step 3 of the test. Which would generate a false positive test result.
Step 2: Add to the cleaned sample some short “primer” sequences of DNA, constructed to be complementary to – and thus able to anneal with or stick to – the specific viral RNA genes to be detected (and, hopefully, not to any other segment of RNA that happens to be in the sample).
Step 3: Add reverse transcriptase enzymes. These are proteins which ‘reverse transcribe’ RNA into strands of complementary cDNA. Any primer stuck to RNA in the sample is used as a starting point for this reverse transcription.
Again successful completion of Step 1 is vital here – this time with regard to complete removal of proteins from the sample. While the kind of reverse transcriptase added by the test kit is thought to need an annealed primer to initiate its reverse transcripton activities (thus ensuring that only those RNA strands with primer stuck to them – that is, only RNA with the SARS-CoV-2 genes being sought – will be reverse transcribed) there do exist in nature reverse transcriptases that do not need any primer to initiate reverse transcription (6,7). These are widely found in viruses called retroviruses (including SARS-‐CoV-‐2 (8)). Retroviruses are ubiquitous, and all of them come with their own reverse transcriptases, which create DNA copies of the retroviral RNA genome, for insertion into the host’s DNA. Thus, if the human nose swab being tested happened to contain a subclinical load of retrovirus – and thus a retroviral reverse transcriptase that did not need a primer to work – this reverse transcriptase could form cDNA off any old RNA that happened to be in the sample. Which again, could easily generate a false positive test result.
Step 4: If (and only if) all has gone according to plan thus far, there are now exactly as many single stranded cDNA copies in the sample as there were SARS-CoV-2 RNA strands in the original sample – and no other DNA. Now the real-time quantitative polymerase chain reaction (qPCR) that gives the test its name is started. An enzyme called DNA polymerase is added, which replicates the cDNA copies. And in order for these replicated DNA copies to be detectable, a probe is also added, which produces an aliquot of fluorescence in the medium every time a DNA replication event happens.
The upshot is that if there was a lot of SARS-CoV-2 RNA in the original sample (and thus a lot of cDNA copies produced in Step 3 of the test), only a few cycles of DNA replication are needed to produce detectable fluorescence in the medium. The less viral RNA there was in the original sample, the more replication cycles are needed to produce detectable fluorescence.
The number of replication cycles needed to produce detectable fluorescence is called the cycle threshold (Ct) of that particular sample. So the higher the cycle threshold, the less virus there was in the original nose swab. Low cycle thresholds indicate more virus.
By global convention, particular instantiations of this test that have produced no detectable fluorescence after 40 or 45 DNA replication cycles are described as negative for the virus. Conversely, any test (using the word now in the sense of any PCR test performed on a particular sample produced from a particular nasal or throat swab) which produces detectable fluorescence after fewer than 40 cycles of DNA replication – any test with a Ct of less than 40 – is deemed positive for SARS-CoV‐2.
In practice, a Ct of 25 or less is regarded as a strong positive and a Ct over 30 or 33 as a weak positive. However, just a positive test – i.e. anything with a Ct of less than 40 – is all that is necessary for reporting of ‘a confirmed case’ of the virus (this despite universal acknowledgement that virus can virtually never be cultured or recovered from test samples with Cts above about 33). While it is often claimed that weak positive tests are repeated, the data giving rise to Table 1 below indicate that in practice this is done – or at least recorded as being done – only occasionally. And importantly, it has never been made clear whether or not weak positive tests are still reported as ‘cases’ in the daily TV announcements of new case counts. Given that anything with a Ct below 40 officially counts as ‘a confirmed case’, one must assume that they are.
All of this notwithstanding though, the question of whether or not a positive test of any cycle threshold actually indicates the presence in the original sample of infectious virus turns out to be a very vexed one. Even the once-‐over-‐lightly description above suggests a dizzying number of points in a generic PCR test at which operational factors quite unrelated to the presence or absence of functional SARS-‐CoV-‐2 in the sample could affect the Ct of any individual instance of the test. And even more pitfalls than those which might be inferred from the preceding description have been published9, 10 by a group of 22 independent scientists who argue that the kind of PCR test launched in 2020 by a publication now known as the Corman-‐Drosten paper is so flawed as to be meaningless. As these workers put it “In light of our re-‐examination of the test protocol to identify SARS-‐CoV-‐2 described in the Corman-‐Drosten paper, we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless”. The huge but largely unreported stoush that resulted from this is well summarised by Sousa (11).
In addition to such mechanistic difficulties, the statistics of the situation also turn out to be far from intuitively obvious. A calculator published by the BMJ12 allows input of the sensitivity and specificity of any sort of test, along with what counter-intuitively turns out to be the other most important factor, the baseline incidence of the condition being tested for, and outputs the numbers of false positives and false negatives produced by the test in question. Hunt (13) plays with this calculator and says [comments in italics added] “First, we conservatively assumed 1% pre-‐test probability of active infection, which is a higher level of active infection than was found in the large vaccine clinical trials. [So this was never an emergency in the first place – fewer than 1% of people even catch this virus, let alone die of it?? ] We also assumed 58% sensitivity and 99% specificity, which are the findings of a recent Cochrane meta-analysis combining 64 published studies of antigen test accuracy, when used to test asymptomatics (Dinnes, J. et al. 2021).
The result in this scenario is 50% false positives (1 false positive for every true positive) – even with a 99% specificity test. There would theoretically be zero false negatives, so the risk of missing actual infections is not at issue.
50% is the same as random chance. In other words, this 99% specificity test can do no better than a coin flip when declaring a positive result. So screening in this scenario is not warranted, because data that is no better than a coin flip is not data – it’s random chance.
However, the situation is much worse than this because neither PCR nor antigen tests are close to a 99% specificity level in practice, for various reasons (Braunstein et al. 2021). Lee 2020 performed a lab analysis of the CDC PCR test accuracy, which was widely used in the first months of the pandemic, and found it had a 70% specificity (i.e. 30% false positives) and 80% sensitivity (20% false negatives). This is because of faulty designs built in to the test from the beginning, as various news accounts from the Washington Post, NPR and ProPublica have since revealed.
This level of inaccuracy matches the CDC’s own internal report that found 33% false results when its PCR test was released in late February 2020, as reported on by National Public Radio (Temple-‐Raston 2020). Intuitively, and in an emergency situation, we may think that a 70-80% accuracy rate is far from perfect but may still be “good enough. ”But this is where common sense and intuition gets us – and the public – into trouble. If we input these figures in the BMJ calculator, we obtain a catastrophic 30 out of 31 false positives. In other words, at a 1% pre-test probability (background prevalence), just one out of 31 positive test results is a true positive. And, again, we have zero false negatives, so the tests are not missing true positives in this scenario.”
Some Local NZ Data
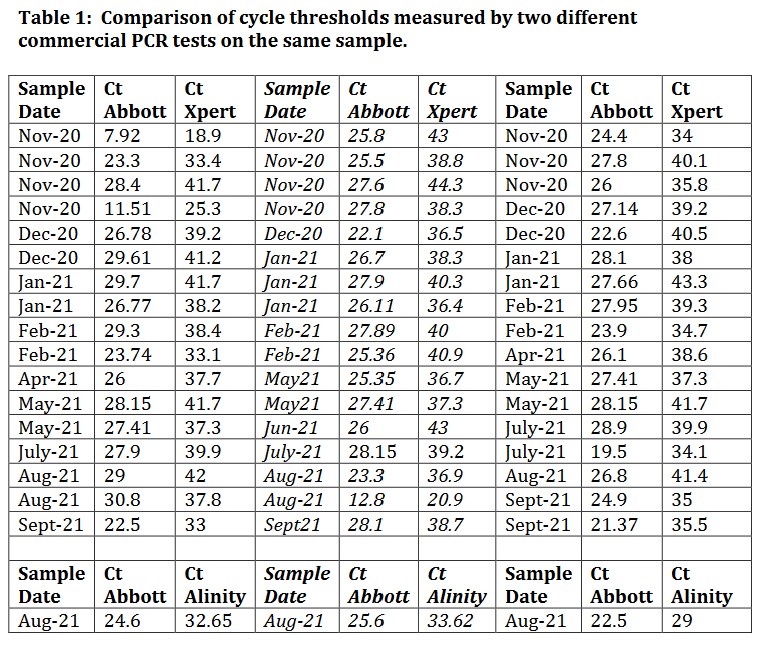
On 1 December 2021, the NZ Ministry of Health was influenced by the New Zealand Ombudsman to reconsider their earlier refusal of an OIA request for the number of PCR tests done in New Zealand that returned a cycle threshold of 35 or above, and released a large tranche of data supplying the cycle thresholds of all positive PCR tests done in New Zealand from August 2020 to September 2021. This is now in the public domain (14). The most interesting fact to emerge from these data turned out to be that illustrated by Table 1.
Most of the two thousand-odd tests for which Cts were reported in the released database were obtained using only one of the several commercial tests available. And, unfortunately as it turns out, the brand of test used for any particular sample was mostly not recorded. But some individual samples were tested twice or more, using two different commercial test kits. As shown in Table 1, this revealed that Abbott tests invariably produced Cts considerably lower than those produced by other brands of test done on the same sample. In other words, Abbott tests tended to inflate the number of ‘strong positives’. This is unlikely to be due to any inability of the other tests to cope with the appearance of new ‘variants’ of the virus, because concern about the reliability of Abbott tests appears to have started in November 2020, well before the arrival of the delta variant in New Zealand. The data at that time already showed unequivocally that Abbott tests always disagreed with Xpert tests (and – data not shown in Table 1 – that Xpert tests produced essentially the same result on any given sample as did all other tests). Yet the data released at the beginning of December 2021 show that the NZ Ministry of Health was still using Abbott tests a full year after their own November 2020 findings (Table 1) that Abbott tests routinely scored samples as ‘strong positive’ which other tests scored as either ‘weak positive’ or frankly negative (Ct 40 or above).
And since the name of the test used is not recorded for the vast majority of entries in the database, it is unclear how many of the ‘strong positive’ Ct results in this database were produced using the suspect Abbott tests.
These data clearly show that samples classified by one brand of test as ‘strong positives’ are often reclassified by a different brand of test as either weak positives or outright negatives (Ct ≥ 40). And given that the brand of test used on most samples is unrecorded, PCR testing in New Zealand would appear to be little more than a lottery.
Worse, the data in Table 1 suggest that the NZ Ministry of Health has known for at least a year that the reliability of the PCR testing they administer is too low to provide any acceptable rationale for the unprecedented mandates enacted on the basis of these tests by the government. Further, the MoH presently not only knows, but says in so many words on their website, that the newer RATs (rapid antigen tests) which nominally detect viral proteins rather than viral RNA, also produce an unacceptably high percentage of false positives – especially in the circumstances in which they are mostly used (“At low levels of prevalence, bulk testing of asymptomatic people and the risk of having a false-‐positive test results will exceed the public health benefit”15 ). Yet as of 15 December 2021, citizens wishing to travel from Auckland to other regions of New Zealand are required to show either (a) a vaccine passport (signifying that the holder has been injected with currently two and in the near future three doses of a new and unprecedented type of ‘vaccine’, demonstrations of the efficacy of which necessarily rely entirely on PCR tests), or (b) evidence of a negative RAT within the previous 72 hours. What happened to Section 18 of the NZ Bill of Rights Act 1990?
Conclusion
Every statement made about this pandemic is ultimately based on the diagnosis of ‘cases’ of covid 19. So the question in the title of this paper becomes rather important. What, after all, is ‘a case’ of covid 19?
As long as the definition remains ‘PCR test with a cycle threshold less than 40’, it must be concluded that the word ‘case’ has lost all real-‐world meaning.
References
1. WHO covid case definition (2020)
https://www.who.int/publications/i/item/WHO-‐2019-‐nCoV-‐
Surveillance_Case_Definition-‐2020.2
2. Adams, G. A beginner’s guide to RT-‐PCR, qPCR and RT-‐qPCR. Portland Press
(2020) DOI:10.1042/bio20200034 Corpus ID: 225662051
3. Department of Biochemistry University of Otago. Coronavirus testing – how
does it work?
https://www.otago.ac.nz/biochemistry/research/otago736925.html
4. Kennedy RF Jr. The real Anthony Fauci: Bill Gates, Big Pharma and the global war on democracy and public health. Skyhorse Publishing N.Y. (2021) 450 pp.
6. Hizi A. The reverse transcriptase of the Tf1 retrotransposon has a specific
novel activity for generating the RNA self-‐primer that is functional in cDNA
synthesis. Journal of Virology (2008) November p. 10906-‐10910
doi:10.1128/JVI.01370-‐08
7. Tuiskunen A, LeParc-Goffart I et al. Self-‐priming of reverse transcriptase
impairs strand-‐specific detection of dengue virus RNA. Journal of General
Virology (2010) 91, 1019-‐10277
8. Zhang L, Richards A et al. Reverse-‐transcribed SARS-‐CoV-‐2 RNA can integrate
into the genome of cultured human cells and can be expressed in patient-‐derived
tissues. PNAS (2021) 118(21) e2105968118.
https://doi.org/10.1073/pnas.2105968118
9. Borger P, Malhotra RK, Yeadon M et al. External peer review of the RTPCR test
to detect SARS-‐CoV-‐2 reveals 10 major scientific flaws at the molecular and
methodological level: consequences for false positive results.
http://doi.org/10.5281/zenodo.4298004
10. https://www.bitchute.com/video/k45zrvEIYkLT/
11. Sousa A. 40-‐cycle RT-‐PCR test for Covid 19: A weapon of mass destruction?
Biomedical Jounral of Scientific and Technical Research (2021) 33(4) 25914-‐
25916 https://biomedres.us/fulltexts/BJSTR.MS.ID.005420.php
12. Watson J and Whiting PF. Interpreting a covid 19 test result . BMJ (2020)
369:m1808 https://www.bmj.com/content/369/bmj.m1808
13. Hunt T. Rapid Response: The “false positive paradox” and risks of testing
asymptomatics. BMJ (2021) 373:n1411
https://www.bmj.com/content/373/bmj.n1411/rr
14. CT Values with Month 3.csv.txt https://fyi.org.nz/request/15879-‐cycle-‐
thresholds-‐of-‐positive-‐sars-‐cov-‐2-‐tests-‐in-‐new-‐zealand?nocache=incoming-‐
67553#incoming-‐67553
15. https://www.health.govt.nz/our-‐work/diseases-‐and-‐conditions/covid-‐19-‐
novel-‐coronavirus/covid-‐19-‐health-‐advice-‐public/assessment-‐and-‐testing-‐
covid-‐19/rapid-‐antigen-‐testing