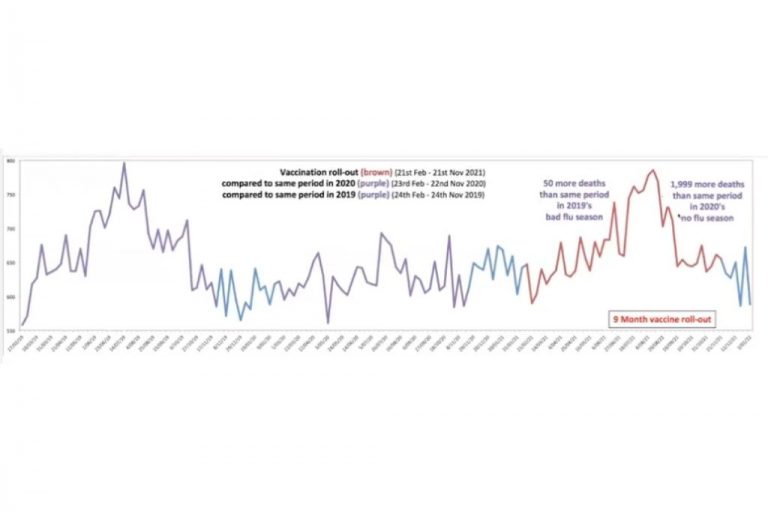
Child Health Doctors Avoid Comirnaty Questions

According to the European Medicines Agency (EMA) Risk Management Plan for BNT162b2, known as Comirnaty, the purpose of Pfizer Study C4591007 is “To assess the safety, tolerability, immunogenicity, and efficacy of the BNT162b2 RNA-based COVID-19 vaccine candidate against COVID-19 in healthy paediatric subjects”. In New Zealand, Medsafe are responsible for evaluating vaccine safety and efficacy before approving or declining consent for use.
Medsafe granted provisional consent for the use of Comirnaty 10mcg for children aged 5 to <12 years old, given as two doses 21 days apart, commencing 17 January 2022. According to Medsafe Corrigendum Provisional Consent, December 2021, consent is granted until 3 November 2023. This is subject to specific conditions, including that Pfizer “Provide the six months analysis data from Study C4591007. Due date: 28 February 2022.”
NZDSOS Questioning Comirnaty Safety Claims
On 17 January 2022, 42 days ahead of the due date of Pfizer’s response to this condition, Dr Jin Russell, a developmental paediatrician at Starship Hospital, endorsed the injection as safe in the presence of the Prime Minister and mainstream media. Since February 2022 an NZDSOS supporter has made repeat attempts by email and telephone to contact Dr Russell seeking details regarding the Pfizer six-month analysis.
We have four simple questions:
- Was the 6 month analysis provided to Medsafe by the due date?
- Was the six month analysis data known prior to the NZ Government rollout of injections for 5 to 11-year-olds?
- Was the due date extended by mutual agreement and if so, on what grounds?
- If the due date was extended, was it safe and responsible to commence the rollout for 5 to 11-year-olds in New Zealand, as an extension suggests either lack of evidence, incomplete evidence, or something else altogether?
No one from the Ministry of Health appears willing to answer these questions. Dr Russell was contacted nearly 4 months ago regarding these, as well as questions about other paediatric related studies and about the ‘buffer’ in the Comirnaty injection being administered to 5 to 11-year-olds in New Zealand. This is not the same buffer used in the clinical trials for this age cohort.
The EMA Risk Management Plan also outlines Pfizer Study C4591036, the purpose of which is “To characterize the clinical course, risk factors, long-term sequelae, and quality of life in children and young adults <21 years with acute post-vaccine myocarditis”. How can Dr Russell be confident that Comirnaty is safe for children when that study will not be complete until 31 October 2025?
One might ask what universe she is living in, given the incalculable reports of post-injection myocarditis in young people globally? At the very least this data is a ‘signal’. How many more young people must take this risk before the study is completed in 2025, and before Comirnaty is withdrawn from human use?
Dr Russell has not responded at all. Is this satisfactory? She asserted at the time of rollout that the only difference in the product for 5 to 11-year-olds was the dose they would receive. That was factually incorrect, otherwise known as misinformation. Where is the transparency? Where is the ‘abundance of caution’? Primum Non Nocere.
An Official Information Act request has been made to establish who knew what and when, including whether Pfizer’s six-month analysis was provided within the time frame and was deemed acceptable by Medsafe. Our representative was informed by the Ministry of Health some time ago that all Pfizer responses to ‘Questions’ in the New Zealand Provisional Consents are confidential.
Medsafe has asked 71 questions of Pfizer since February 2021. The fact that all consents remain provisional raises concern. It is also pertinent that the original Provisional Consent was extended for another two years on 28 October 2021. We refer you to the Risk Management Plan document pages 160-167 for details on documented risks and ongoing studies into missing risk information associated with Comirnaty.
Expert advisors to the New Zealand government, deemed as reliable and free from bias or conflicts of interest, wield influence over the general public. Should we not be able to request answers from them when they assert safety of a product without the necessary information to make that assertion? Are they not accountable for their advice and public statements?
Given the global reporting of adverse outcomes since the rollout of this experimental gene therapy injection and the associated risks, the latest warning by Geert Vanden Bossche is of concern. It is exasperating to see the New Zealand media featuring experts such as Dr Jin Russell and GP Dr Nikki Turner, who we named in Our Complaint to the Broadcasting Standards Agency in March 2022, waxing lyrical from the Podium of Truth about a fourth Comirnaty injection.
How can these individuals and their institutions be held to account?