Te Whatu Ora’s Planned Covid-19 Vaccination Policy: An Informative Critique

Comment on Te Whatu Ora DRAFT Pre-employment Occupational Health Vaccination Policy version 0.6
I am a New Zealand specialist who was employed fulltime at a DHB prior to being fired for failing to comply with COVID-19 vaccine mandates on 15 November 2021. I contracted COVID-19 in March 2022.
I have a Doctor of Medicine degree and have held an academic appointment. I have multiple refereed publications (about 20% of which were in the last 3 years) and multiple reviews and chapters. I am an examiner for specialist exams.
I have been sent a draft copy of the Te Whatu Ora Vaccination Policy. Whilst I am not employed by Te Whatu Ora I have been asked by a locum agency to do locum work from October 2022 in hospitals where no COVID-19 vaccinated SMO locums could be found to cover critical vacancies. I have done a number of locums since then and am getting requests every two weeks or so to do work several months in advance. As the Vaccination Policy will apply to locums I am offering my comments on the Policy.
Page 5 of the document states that Te Whatu Ora has received advice on specific diseases and vaccinations from IMAC and the NZ Ministry of Health website. The full list of documents consulted has not been given. As a result, I will restrict my comments to drawing your attention to recent papers or significant information related to the COVD-19 vaccines which will not have been considered by either IMAC, the NZ MOH, or by Te Whatu Ora in the writing of the version of the draft which I have access to.
1. Rationale for COVID-19 vaccination for Health Care Workers
The document acknowledges that the vaccinations do not prevent transmission of disease and that SARS-CoV-2 is now widespread in the community so the aim of vaccinating staff is to limit the impact of the disease on workers so they require less time off-work.
1a. Data shows that risk of COVID-19 infection increases with the number of vaccination doses administered and does not prevent symptomatic disease in most
Shrethsa et al from the Cleveland Clinic posted a paper on medRxiv in December 2022. They used retrospective data from 51,011 Clinic employees to investigate the effectiveness of the bivalent vaccine.
The authors found an ‘unexpected’ association between increased risk of COVID-19 and higher number of vaccination doses as shown in Figure 2 of their paper.
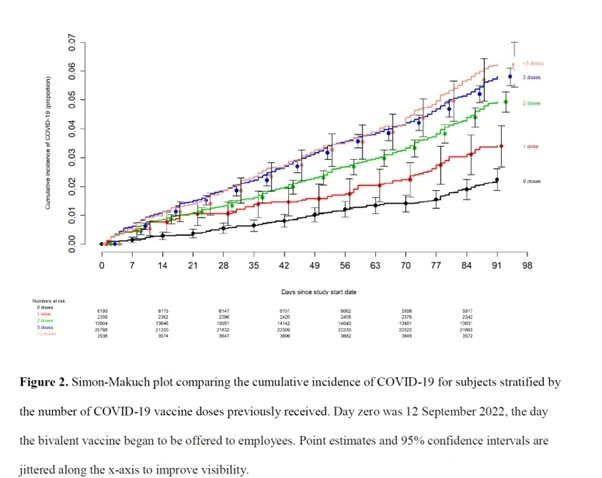
In the discussion they note that two other studies have similar findings – one showed an increase in Omicron in those who had received three doses of COVID-19 vaccination as opposed to two doses, and another showed an increased risk of COVID-19 after two doses compared to one dose. They suggested that ‘it is important to examine whether multiple vaccine doses given over time may not have the beneficial effect assumed’.
Marking et al (September 2022) from Sweden looked at breakthrough infections in triple vaxxed healthcare workers (31 to 37 days after third dose). They found an infection rate of 22% with only 10% of people being completely asymptomatic. They also found that while the viral load peaked on day 3, live virus (based on PCR cut off) could be found for up to 9 days.
The NEJM published a Letter (letter) from Boucau et al in July 2022 which showed vaccinated and boosted people took up to 15 days to clear the virus.
These studies show that infection risk increases with the number of vaccines, and the vast majority (90%) of infected boosted healthcare workers experienced symptomatic infection and carry live virus for 9 to 15 days. This data suggests that requiring healthcare workers to be vaccinated and boosted will increase their risk of symptomatic infection and will thus increase the risk to patients and co-workers.
1b. Data show that repeat doses of COVID-19 vaccination induce a switch to IgG class 4 antibodies and immune tolerance
Two recent studies seem to go some way to explaining the increasing risk of infection with multiple vaccination doses.
Irrang et al looked at the effect of repeated COVID vaccinations on IgG antibodies.
I quote from the Abstract.
Shortly after the initial two mRNA vaccine doses, the IgG response mainly consists of the pro-inflammatory subclasses IgG1 and IgG3. Here, we report that several months after the second vaccination, SARS-CoV-2-specific antibodies were increasingly composed of non-inflammatory IgG4, which were further boosted by a third mRNA vaccination and/or SARS-CoV-2 variant breakthrough infections. IgG4 antibodies among all spike-specific IgG antibodies rose on average from 0.04% shortly after the second vaccination to 19.27% late after the third vaccination. This induction of IgG4 antibodies was not observed after homologous or heterologous SARS-CoV-2 vaccination with adenoviral vectors.
I quote from the body of the paper.
However, the large number of breakthrough infections caused by the Omicron variant indicates that current vaccination regimens do not confer sterilizing protection. Once infection is established, Fc-mediated effector functions become more relevant to clear viral infections. Systemic serology approaches have even revealed that different antibody functions can contribute to various degrees to protection dependent on the viral pathogen, as shown for influenza viruses, RSV or SARS-CoV-2 (37–40). Passive immunization studies in animal models have further demonstrated that the degree of protection achieved by the application of monoclonal antibodies depends on their IgG subclass (41–44). In this regard, IgG4 is considered as an anti-inflammatory IgG with low potential to mediate Fc-dependent effector function such as ADCC or ADCP (20, 45).
High levels of antigen-specific IgG4 have been reported to correlate with successful allergen-specific immunotherapy by blocking IgE-mediated effects (46). In addition, increasing levels of bee venom-specific IgG4 have been detected in beekeepers over several beekeeping seasons and finally even became the dominant IgG subclass for the specific antigen, i.e. phospholipase A (PLA).
In January a German group published similar findings (Buhre et al, 2023) and I quote.
Very interestingly, two mRNA immunizations as well as one AZD1222 immunization with an mRNA booster, in particular with the mRNA-1273 vaccine, induced long-term anti-S1 IgG4 responses – the IgG subclass with inhibitory effector functions – in naïve subjects. In contrast, we could not observe such an increase upon two immunizations with the AZD1222 vaccine in naïve individuals up to day 270, suggesting that only mRNA vaccines generate detectable long-term IgG4 responses at least until day 270.’
These studies both show that immune tolerance develops with increasing doses of mRNA vaccination over some months. Immune tolerance prevents rapid clearance of infection and prevents the formation of lasting neutralising immunity thus making affected people suffer repeat reinfections. The repeat infections might be accompanied with mild symptoms because immune tolerance fails to elicit a strong reaction and stop viral replication. However, the virus is left to proliferate unopposed and thus can cause damage to the host. Could this be why we see so many reports of people who pass away unexpectedly after a ‘mild’ COVID-19 illness?
1c. Other implications of the change in immunoglobulins after COVID vaccination
Effect on cross reactivity with other viruses.
SARS-CoV-2 is an RNA respiratory virus. So are a lot of other viruses. We know there is cross-reactive immunity between SARS-CoV-2 and other related corona viruses which confer protection against COVID-19. Irrang et al also noted this.
Systemic serology approaches have even revealed that different antibody functions can contribute to various degrees to protection dependent on the viral pathogen, as shown for influenza viruses, RSV or SARS-CoV-2.
Chaisawangwong et al (2022) found correlation between SARS-CoV-2 and Influenza A virus specific CD8 T cell responses. They point out that this might also apply to other viruses like RSV. They think this might be a great thing – mRNA COVID injections can provide protection against other respiratory viruses. But what if the reverse happens – what if the switch to IgG4 with repeated injections which causes immune tolerance to SARS-CoV-2 also causes immune tolerance to other respiratory viruses?
Effect of immune tolerance on cancer cells and auto immunity.
The switch to IgG4 is accompanied by a significant change in the proportions of IgG subtypes in serum. Irrang et al showed that the proportion of IgG1 fell from 88.98% after the second dose to 72.58% after the third. What is the effect of a lower percentage of IgG1? This is particularly important as we know that IgG1 is important for tumour suppression. Are we seeing an increase in cancers or cancer recurrence?
In October 2022 Loaker et al published data which showed an increase in PD L1 surface expression on peripheral blood granulocytes and monocytes after COVID vaccination. PD-L1 I expression tells T cells to ignore tumour cells. When PD-L1 is inhibited, for example by the PD-L1 inhibitor pembrolizumab (Keytruda) T cells seek and destroy malignant melanoma cells.
The COVID vaccine induced an increase in PD-L1 expression so it may have the opposite effect of Keytruda on T cells. If so we might see an acceleration of malignant melanoma and other PD-L1 mediated tumour types. You can read a full explanation about this here.
Irrang et al showed the proportion of IgG4 increased from 0.04% to 19.27%. The degree of switch to IgG4 is dose dependent but we don’t know how long it lasts for and what the downstream implications of such a change is for the immune system. IgG4 acts via its effect on T regulatory cells. T regulatory cells are what prevent our immune system attacking our own cells. Changes in the proportion of different types of T regulatory cells may affect our ability to prevent autoimmune disorders. Furthermore, there is a condition called IgG4 related disease which causes a fibro-inflammatory process in different organs or locations. Changes in PD-L1 expression has also been found to occur in IgG4 related conditions. See here for a more detailed explanation. Are we seeing more autoimmune diseases or exacerbation of autoimmune conditions and what about conditions related to fibrosis in organs?
2. Health and Safety Risk Assessment
Page 4 of the draft policy states that Te Whatu Ora Occupational Health Services provide a health and safety risk assessment service for workers.
2a. What are the risks of COVID-19 infection?
Pezzullo et al (2023) from Stanford identified 40 eligible national seroprevalence studies covering 38 countries with pre-vaccination seroprevalence data. For 29 countries (24 high-income, 5 others), publicly available age stratified COVID-19 death data and age-stratified seroprevalence information were available and were included in the primary analysis. The median IFR by age groups is as follows.
0.0003% at 0–19 years
0.002% at 20–29 years
0.011% at 30–39 years
0.035% at 40–49 years
0.123% at 50–59 years
0.506% at 60–69 years.
These IFRs will be from infection with the wild strain, alpha and delta variants whereas it is accepted that the Omicron strains lead to much milder clinical disease.
The authors point out that these IFRs are lower than previously reported and also note that good serological data shows even lower IFRs. They quote a national study of blood donors in Denmark which estimated an IFR of only 0.00336% for people <51 years without comorbidity, and 0.281% for people aged 61–69 years old without comorbidity.
This data shows a very low risk of death from COVID-19 for the vast majority of people likely to be employed by Te Whatu Ora. This leads us to the second part of the health and safety risk assessment.
2b. What are the risks of COVID-19 vaccines?
The most astounding aspect of this vaccination policy is the complete absence of any reference to or discussion of the risks of COVID-19 vaccines.
i. Myocarditis
The risk of myocarditis after vaccination is now accepted and has resulted in vaccine doses being limited to a total of 3 doses for those under 30 without risk factors in many countries including NZ.
NZ data. A Lancet preprint from Walton et al (2023) has just been released. This paper reports the incidence of 12 adverse events for which hospitalisation occurred within 21 days of either a first or a second dose of the Pfizer vaccine. The incidence was compared to the background rate observed in 2014 to 2019.
Increased rates of myo/pericarditis were seen within 3 weeks of vaccine dose with an overall risk difference of 1.6 after the first dose and 3.2 after the second dose. The highest incidence ratio rate was 25.8 in the 5 to 19 year age group after the second dose. The authors stated this was consistent with an increase of 5 cases per 100,000.
It is notable (but not discussed) that the overall risk difference doubles between doses one and two for people aged 20 to 59 and trebles for people ages 5 to 19. This trend suggests that there could be a further increase in risk after each successive dose of the vaccine. The data shows a nearly 5 times higher risk of hospitalisation for myo/pericarditis for 5 to 39 year olds in the three weeks following their second Covid-19 vaccination. That will not include people who do not report symptoms or people who develop symptoms after 3 weeks. (The study also found an increased risk of hospitalisation for an acute kidney injury within 3 weeks of an mRNA vaccine dose. The risk difference also increased from 19.2 after the first dose to 23.1 after the second.)
The authors state that the risk of myocarditis after COVID-19 infection is still higher than after vaccination. This is not what the largest population based study of the incidence of myo/pericarditis has found (Tuvali et al 2022). This Israeli study used data from before the vaccine roll out in Israel and found no difference in the incidence of this condition in 196,992 unvaccinated adults who had COVID-19 compared to 590,976 age and sex matched controls without COVID-19.
It is quite remarkable that despite the fact that the vaccine related myocarditis risk became apparent in mid-2021, the only two prospective studies to detect myocarditis published to date are on teenagers and were done in Thailand and Taiwan.
Mansanguan et al (2022) followed 301 adolescents after their second dose of Pfizer. Data including demographics, symptoms, vital signs, ECG, echocardiography and cardiac enzymes were collected at baseline, Day 3, Day 7, and Day 14 (optional) using case record forms.
Seven participants (2.33%) exhibited at least one elevated cardiac biomarker or positive lab assessments. Cardiovascular effects were found in 29.24% of patients, ranging from tachycardia, palpitation, and myopericarditis. Myopericarditis was confirmed in one patient after vaccination. Two patients had suspected pericarditis and four patients had suspected subclinical myocarditis.
Chiu et al (2023) performed an ECG screening study during the second dose of BNT162b2 vaccines. Serial comparisons of ECGs and a questionnaire survey in 4928 high school students was used. In total, 763 students (17.1%) had at least one cardiac symptom after the second vaccine dose, mostly chest pain and palpitations. The depolarization and repolarization parameters (QRS duration and QT interval) decreased significantly after the vaccine with increasing heart rate. Abnormal ECGs were obtained in 51 (1.0%) of the students, of which 1 was diagnosed with mild myocarditis and another 4 were judged to have significant arrhythmia. None of the patients needed to be admitted to hospital and all of these symptoms improved spontaneously. The incidence of significant arrhythmias and myocarditis was 0.1% in this study.
Mansanguan et al (2022) showed 2/100 positive biomarker or lab assessment, 1/60 with myocarditis or subclinical myocarditis. After two doses.
Chiu et al (2023) showed 1/1000 myocarditis and significant arrythmia (no troponin levels done). After two doses.
Data suggesting a 2.8% incidence of subclinical myocarditis in healthcare workers following the booster was reported in a presentation by Prof. Christian Mueller (Basel, Switzerland) at the European Society of Cardiology Congress in August 2022: “Myocardial Inflammation/Myocarditis After COVID-19 mRNA Booster Vaccination.” You can read a discussion of the findings here or in a video here.
777 healthcare workers of average age 37 received troponin assays on Day 3 after a Pfizer booster.
40 had increased troponins – the researchers stated that in 18 of them, causes other than the vaccine were identified that could explain the elevated troponins (but don’t say what), in the remaining 22 no cause other than the vaccine was implicated. They investigated everyone with an elevated troponin.
2.8% had myocardial lesions, 3.7% in women and 0.8% in men. Was this unexpected female predominance due to the factors that lead them to find another possible cause for troponin rise in 18 people?
These people were retested on day 4 and in almost all cases their troponin levels fell. The researchers noted that this could mean the troponin levels were higher on days 1 to 3 following the booster.
Their data also suggests that everyone in the study had a small elevation in their troponin levels. They found a control for every person in the study and compared their troponin levels to the day 3 study levels. They produced graphs of the cumulative distribution curve for men and women which shows the curve in the study participants was shifted to the right as shown in the following figure from their presentation.

The risk of death or serious disease due to infection for a generally healthy person under 40 and probably 50 is so small as to be negligible. Yet the risk of subclinical myocarditis after two doses is 2% in teenagers and 2.8% in adult healthcare workers after a booster. What happens after a 4th dose??
Australia and other countries have stopped offering the booster to those under 30 due to their risk of adverse events. Denmark has now ceased to offer mRNA COVID-19 vaccinations to anyone under the age of 50 as it would be better for people to get natural antibodies. Based on this data Te Whatu Ora cannot possibly recommend healthcare students and healthcare workers have the primary course, a booster and any subsequent boosters.
ii. Other serious adverse events
In December 2022 Dr Kerryn Phelps, previous President of the AMA made a submission to the Australian Parliament’s Long Covid Inquiry which made headline news around the world. She and her partner have both suffered vaccine injuries following the Pfizer injection and have discovered many others with evidence of harm but regulatory censoring of doctors who raise concerns about vaccine injuries is suppressing the information. In her submission she quotes German pharmacovigilance data from the Paul Ehrlich Institute which found evidence of serious reaction in 0.3 per 1000 shots. Professor Phelps points out that as most Australians have now had at least one booster the incidence of serious adverse reactions could exceed 1 in 1000 people.
Fraiman et al (September 2022) performed a secondary analysis of serious adverse events reported in the placebo-controlled, phase III randomized clinical trials of Pfizer and Moderna mRNA COVID-19 vaccines in adults.
Using a prespecified list of AESI identified by the Brighton Collaboration, higher risk of serious AESI was observed in the mRNA COVID-19 vaccine group relative to placebo in both the Pfizer and Moderna adult phase III trials, with 10.1 (Pfizer) and 15.1 (Moderna) additional events for every 10,000 individuals vaccinated.
In addition, this analysis identified a 36% higher risk of serious adverse events in vaccinated participants in the Pfizer trial: 18.0 additional SAEs per 10,000 vaccinated (95 % CI 1.2 to 34.9).
This analysis found higher risks of total serious adverse events compared to the FDA reviews because the FDA reported the number of people with serious adverse events but many had two or more events. In addition, this analysis was based on the study population with median follow-up 2 months after dose 2 (minus 120 HIV-positive participants), of which 98.1 % had received both doses. The FDA’s analysis of SAEs however, included thousands of additional participants with very little follow-up, of which the large majority had only received 1 dose.
The authors point out that further analysis of adverse events is hampered by lack of individual patient data.
Note that the Fraiman data is consistent with the German data which suggests 0.6 per 1000 people will experience serious adverse events after two doses.
iii. Problems with the UK data for vaccine efficacy
Vaccine efficacy data requires accurate data on the numbers of both vaccinated and unvaccinated people. You will know that for some time data for NZ was reported for only the 4.2-odd million people with recent interactions with the health care systems rather than the population of around 5.2 million. We heard reports of 95% of the 4.2 million being vaccinated for COVID-19 applied erroneously to the whole country.
It seems that the problem of defining the unvaccinated population numbers occurs in other countries too.
In November 2022 Professor Norman Fenton and colleague Martin Neil wrote a complaint to the UK Statistics Regulator in which they pointed out errors and inconsistency in the ONS vaccine surveillance mortality reports. A link to the letter can be found here.
In summary they provided data to show that there were misclassification and missing deaths, with: a) gross underestimation of the population proportion unvaccinated, and b) mortality rates that are both nonsensical in various categories and completely incompatible with historical rates. People were not counted as vaccinated until 2 weeks after their last dose. Any deaths prior to this were attributed to the unvaccinated category (or to the preceding dose category).
They asserted that all of the anomalies in the dataset introduce bias in favour of analyses supporting vaccine ‘safety and efficacy’. The fact that these data are being used as continued justification for the efficacy and safety of the covid vaccines is therefore now a matter of national concern and scandal.
In January 2023 Professor Fenton received a reply from the Regulator which you can read here. The Regulator agreed that the ONS underestimates the true population of the unvaccinated and that the Deaths by Vaccination Status publication does not provide information on vaccine effectiveness and safety and should not be used to do so.
Professor Fenton’s full report on the ONS vaccine mortality surveillance report for England (for the period 1 Jan 2021 to 31 May 2022) can be found here.
Breaking news from the UK 26 Jan 2023: no more boosters to under 50 year olds.
The UK The Joint Committee on Vaccination and Immunisations (JCVI) has advised the government that it should no longer offer booster doses to anyone under 50 from 12 February 2023.
Similarly, the JCVI is advising that the primary course COVID-19 vaccination should move, over the course of 2023, towards a more targeted offer during vaccination campaigns to protect those persons at higher risk of severe COVID-19. The report and Appendix 1 can be viewed here.
The main rationale given is that the number needed to vaccinate to prevent serious hospitalisation for low risk groups is very large (for example 932,500 low risk 40 to 49 year olds to prevent one serious hospitalisation). But their data shows that the number needed to vaccinate to prevent serious hospitalisation even in the at risk group with the first booster ranges from one in 43,500 (20 to 29 years) to one in 61,000 (50 to 59 years). This is admission of vaccine failure to prevent serious disease. If we use the German data around 1/1000 people will suffer a serious adverse event after three doses. This means that in the risk group between 43 and 61 people could have suffered a serious adverse event to prevent one case of serious hospitalisation (defined as ICU admission or need for oxygen). They have known this since October 2022 yet pushed boosters until late January 2023.
iv. CDC analysis of the VAERS database
The CDC conducted an analysis of adverse events reported in the VAERS system for the Pfizer and Moderna covid vaccines for the period Dec. 14, 2020, to July 29, 2022. They refused to make the results of their analysis public. Zachary Stieber of the Epoch Times obtained the results through a Freedom of Information Act request. He made the information available to several researchers, one being Dr Josh Guetzkow and another being Professor Fenton’s group.
Dr Guetzkow’s analysis dated January 5, 2023 can be found at this link.
This is a summary of some of the data found.
CDC’s VAERS safety signal analysis based on reports from Dec. 14, 2020 – July 29, 2022 for mRNA COVID-19 vaccines shows clear safety signals for death and a range of highly concerning thrombo-embolic, cardiac, neurological, hemorrhagic, hematological, immune-system and menstrual adverse events (AEs) among U.S. adults.
There were 770 different types of adverse events that showed safety signals in ages 18+, of which over 500 (or 2/3) had a larger safety signal than myocarditis/pericarditis.
The CDC analysis shows that the number of serious adverse events reported in less than two years for mRNA COVID-19 vaccines is 5.5 times larger than all serious reports for all vaccines given to adults in the US since 2009 (~73,000 vs. ~13,000).
Twice as many mRNA COVID-19 vaccine reports were classified as serious compared to all other vaccines given to adults (11% vs. 5.5%). This meets the CDC definition of a safety signal.
v. Excess deaths
Most western countries have reported excess deaths though the COVID-19 pandemic. However rather than reducing in 2022 with the combination of vaccines, less virulent Omicron strains and widespread community infections, excess deaths are continuing. This can be illustrated in the UK by reference to the UK Institute and Faculty of Actuaries who publish frequent UK mortality analysis through its publicly available mortality monitor.
On 17 January they reported that 2022 had the worst second half mortality since 2010.
31,000 excess deaths occurred in 2022 – 4.5% higher than the 2019 pre-pandemic year but 7.8% lower than 2020 and 2.2% lower than 2021.
However, of note is the fact that compared to 2019 deaths were 2.5% higher for ages 75 to 84 but 7.8% higher for ages 20-44.
Furthermore, 26,300 excess deaths occurred in the second half of 2022 compared to 4,700 in the first six months. This is the highest number of excess deaths in the second half of any year since 2010. More than 7000 excess deaths occurred in the three weeks to 6 January 2023.
The number of deaths registered in England and Wales in week 1 of 2023 was 3437 higher compared to mortality in the first week of 2019, a 30% increase. Only 739 deaths had COVID-19 mentioned on the death certificate.
If COVID is not the driver of excess deaths what is? And why have deaths in young working-age people increased by 7.6% in 2022?
Ed Dowd is a former Blackrock Financial investment manager who was the first to draw attention to US actuarial data showing excess deaths in the working age population. This has now been widely reported in the US. See the following article for example. In depth analysis of Excess deaths can be found at Dowd’s group and in various Substack articles, for example this one which reports a strong correlation between excess mortality and booster rates in different regions in Germany P= 0.031.
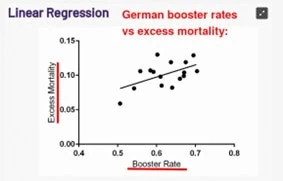
Reference: Covid Boosters Are Killing Germans
Conclusion
The data presented here is just a fraction of the data coming in now on an almost daily basis which show the mRNA COVID-19 vaccines are not only unable to prevent illness, but rather repeated doses increase susceptibility to infection and illness. The proportion of excess deaths in highly vaccinated countries is around 15% to 20% and is increasing with the passage of time. The mRNA COVID-19 injection is quite clearly the most dangerous substance ever to be advertised as a vaccine in history. The UK decision to follow Denmark and walk slowly backwards from a mass COVID-19 vaccination programme is highly significant, especially given the weight placed on UK data in the preparation of the draft guidelines. I urge you to not only remove any requirement for any COVID-19 injections from Te Whatu Ora policies but to also advise the Minister of Health that use of these preparations must be ceased immediately. When you look at the data there is simply no alternative.





